This compendium focuses on functionalised sugar amino acids SAAs and their 3- to 6-membered nitrogen heterocyclic and carbocyclic analogues. Interestingly it has been estimated that 1540 of all PPIs are mediated by a short linear peptide.
9 12 and Nicoloau et al.
. However the synthetic strategy is flexible and convergent and in principle allows for the display of homo- or hetero-dimeric. Design and synthesis of new classes of heterocyclic C-glycoconjugates and carbon-linked sugar and heterocyclic amino acids by asymmetric multicomponent reactions AMCRs. Computational techniques play a key role to design and develop the amino acid-based therapeutics such as proteins peptides and peptidomimetics.
Dondoni A Massi A Acc Chem Res 397451-463 01 Jul 2006. Peptides can be rationally designed based on the natural sequences that mediates PPI in the proteins and therefore can mask a critical part of the. To obtain BTD peptidomimetics with higher predictive activity we alternatively selected the.
Among the analogs of compound 18 compound 32 a cyclic d-amino acid-containing peptidomimetic was found to have an IC 50 value in the nanomolar range in HER2-overexpressing cancer cell lines. To improve the stability of the peptidomimetic d-amino acids were introduced into the peptidomimetic and several analogs of compound 18 were designed. Methods such as phage display library peptide synthesis and computational design are described.
A medicinal chemistry approach where parts of the peptide are successively replaced by non-peptide moieties until getting a non-peptide molecule and a biophysical approach where a hypothesis of the bioactive form of the peptide. Certain other properties such as receptor selectivity or potency often can be substantially improved. This study gives further support to a binding mode similar to.
Although no drug molecule has been developed a number of novel potent ligands for receptors have been identified. Two amino groups allow for the attachment of a diverse array of peptidomimetics via amide bond formation. Request PDF Design Synthesis Conformational Analysis and Application of Azabicycloalkane Amino Acids as Constrained Dipeptide Mimics In the field of.
37 Amino acid variants with unsaturated side chains have also been described. Scaffolding had been used and proven in the 1980s when Hirschmann et al. This article reviews the recent applications of β-amino acids in the.
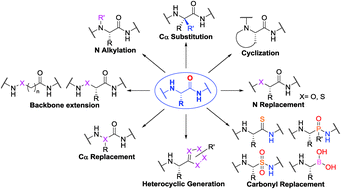
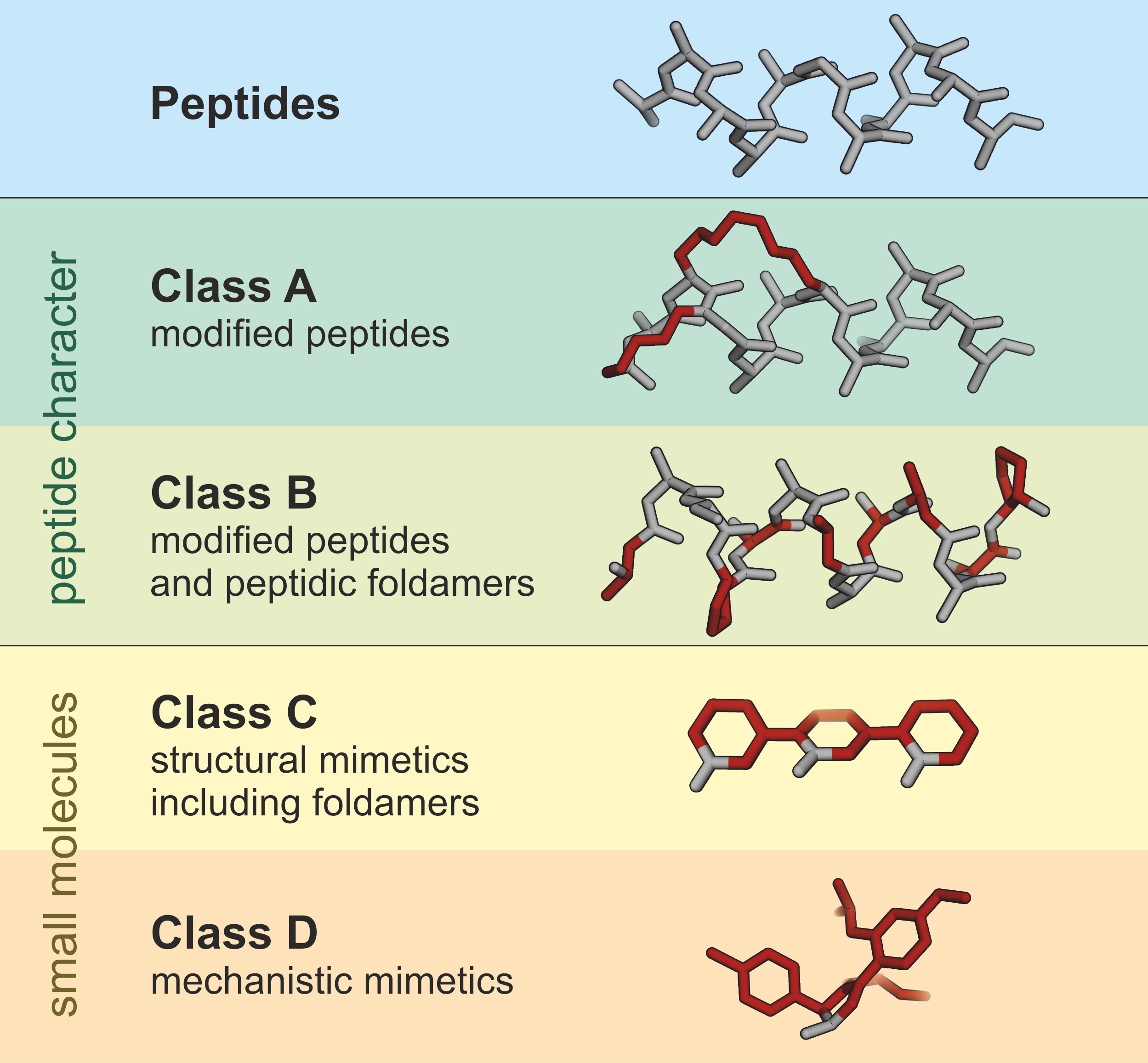
Peptides and peptidomimetics modified peptides on the other hand are perfect candidates to target PPIs. Recent developments in the solid phase synthesis of prospecting combinatorial libraries syntheses of pyranose based sugar amino acids SAAs and other scaffolding for peptidomimetics are included. The use of β-amino acids as peptidomimetics has emerged in recent years with significant potential in a number of applications.
The final part deals with amino acids in combinatorial synthesis. Self-structural organizations such as turns helices sheets and loops can be accessed by chemical modifications of amino acids or peptides. 13 described an approach to design of peptidomimetics wherein the entire amide backbone of a β-turn is replaced by novel scaffoldings devoid of amide bonds or isosteric replacements described in more detail in the section Carbohydrates as Scaffolds in the Design.
Peptidomimetics represent an important field in chemistry pharmacology and material science as they circumvent the limitations of traditional peptides used in therapy. Full PDF Package Download Full PDF Package. Basically there are two different approaches to design peptidomimetics.
To enable the rational design of new peptidomimetics with high bitter taste sensitivity we developed an in-house C program to map out amino acid preferences at different sequence positions based on the GA-PLS coefficients along with the scores of 615 amino acids. The approach was exemplified by the linking of α-helical mimics of the i and i4 positions to replicate the structure of an α-α hairpin. Originally planned as a six volume series Amino Acids Peptides and Proteins in Organic Chemistry now completes with five volumes but remains comprehensive in both scope and coverage.
38 Further development of such noncanonical amino acids offers a. Up to 10 cash back Abstract. The incorporation of β-amino acids has been successful in creating peptidomimetics that not only have potent biological activity but are also resistant to proteolysis.
The design of even smaller peptidomimetics seems possible as an SAR study for class II reports nanomolar affinity of a N- and C-terminally capped tetrapeptide 20 including the unnatural amino acids cyclohexylalanine and norvaline at position 1 and 3 respectively Cunningham et al 1997. Practical stereoselective synthesis of conformationally constrained unnatural proline-based amino acids and peptidomimetics. Peptidomimetics are designed to circumvent some of the problems associated with a natural peptide.
Amino acid-based drugs are a group of biologic-based therapeutics that can effectively combat the diseases caused by drug resistance or molecular deficiency. Stability against proteolysis duration of activity and poor bioavailability. The main benefit of using SAAs and their related nitrogen and carbon congeners in the production of peptidomimetics and glycomimetics is that their properties can be readily.

Computer Aided Design Of Amino Acid Based Therapeutics A Review Dddt

Peptidomimetics Via Modifications Of Amino Acids And Peptide Bonds Chemical Society Reviews Rsc Publishing

Pdf Chemical Modifications Designed To Improve Peptide Stability Incorporation Of Non Natural Amino Acids Pseudo Peptide Bonds And Cyclization

Solid Phase Synthesis Of Peptidomimetics With Peptide Backbone Modifications Abdildinova 2021 Asian Journal Of Organic Chemistry Wiley Online Library




0 comments
Post a Comment